Abstract
Background: There remains a critical need to identify untreated HR FL patients (pts) who are at increased risk of early progression and death with conventional treatment. Data examining baseline PET has been reported to be prognostic in FL, however, there remains a paucity of data analyzing this in the context of bendamustine/rituximab (BR) induction followed by maintenance rituximab (MR). We examined the prognostic impact of baseline PET metabolic imaging from the randomized BIONIC study for pts with untreated HR FL.
Methods: The BIONIC study conducted by the ECOG-ACRIN Cancer Research Group enrolled untreated grade I/II or IIIa FL pts with HR disease defined as GELF high tumor burden and/or FLIPI score of 3-5. Pts were randomized to: a) standard BR induction x 6 followed by maintenance rituximab (MR) q 2 months x 2 years vs b) bortezomib + BR x 6 followed by MR q 2 months x 2 years vs c) BR x 6 followed by q 2 months MR x 2 years + lenalidomide given concurrently with the first year of MR. Survival analyses showed no significant differences in 4-year progression-free survival (PFS) or overall survival (OS) for any study arm (Evens AM et al, ASH 2017). All pts were thus pooled for the purpose of analyzing baseline PET imaging and other characteristics and for correlation with clinical outcomes. Kruskal-Wallis & Wilcoxon Rank Sum test were used to assess differences in distribution of: metabolic measurements (SUVmax and SUVmean of the dominant mass (i.e., the mass/lesion with the highest SUV uptake); metabolic tumor volume (MTV); and total lesion glycolysis (TLG) by categorical variables); induction treatment response; and end-of-induction (EOI) PET response vis-à-vis Deauville scoring (1-3 = negative). Cox proportional hazards regression was used to correlate these metabolic measurements with PFS & OS and multivariable analyses were conducted. An optimal cutoff threshold (grouping into 'High' or 'Low' categories) was used for the metabolic measurements that were significantly associated with PFS & OS.
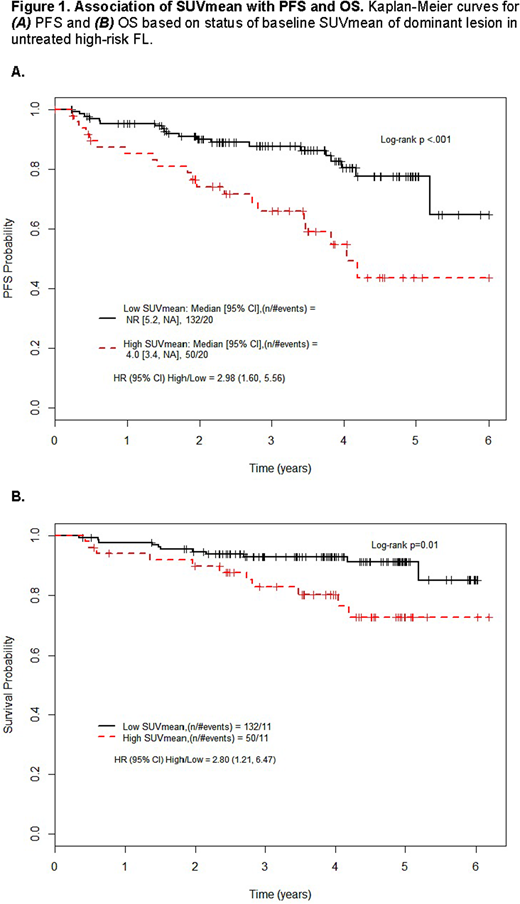
Results: Metabolic data were received and measurements were analyzed centrally on 182 (71%) of 257 eligible pts. The EOI overall response rate (ORR) for all pts on study was 92% with a complete remission (CR) rate of 73%. Additionally, 12% of pts experienced early progression of disease (POD) within 2 years. SUVmax was borderline for association with PFS (P=0.06) and MTV and TLG were not associated with PFS or OS. We demonstrated a statistically significant linear relationship between continuous SUVmean score with PFS and OS, and thereafter, used the minimum p-vlaue approach to determine the optimal cutoff threshold of 7.03 to group pts into 'High' and 'Low' risk groups; with this threshold, baseline SUVmean of the dominant mass was categorized as high risk in 27% pts. The 4-year PFS rates for SUVmean low vs high groups were 80% (95% CI 72, 90%) vs 55% (40, 74%), respectively, P<0.001; and the corresponding 4-year OS rates were 93% (89, 98)% vs 80% (95% CI 69, 93%), respectively, P=0.01 (see Fig. 1). Sensitivity analyses showed no differences in baseline SUVmean by study arm, GELF or FLIPI. The only clinical characteristic that varied among patients with low vs high baseline SUVmean was Black race (0.8 vs 8.0%, respectively, Chi-square P=0.08); there were no other differences based on age, sex, stage, FLIPI, GELF status, ECOG PS, LDH, or # of extranodal sites. In addition, baseline SUVmean correlated with EOI PET (negative EOI PET with baseline SUVmean Low vs High: 81% vs 62%, respectively, P=0.03). In multivariable analyses, both baseline SUVmean and EOI PET remained significant (SUVmean: PFS HR 2.62 (95% CI 1.40-4.92), P=0.001, OS HR 2.37 (95% CI 1.01-5.56), P=0.02; EOI PET PFS HR 4.70 (95% CI 2.74-9.54), P<0.001, OS HR 2.78 (95% CI 1.18-6.56), P=0.01). Finally, baseline SUVmean was also strongly predictive of FL pts who experienced early 2-year POD (Chi-square P<0.001).
Conclusions: High baseline SUVmean of the dominant mass was a strong independent prognostic factor for survival for untreated HR FL pts treated with BR-based induction with rituximab maintenance and significance was independent of FLIP or GELF criteria. Collectively, this represents a novel and simple method to identify FL pts at increased risk of early POD and poor survival with standard therapy. Continued investigation of combined PET imaging analyses and other correlative studies to enrich prognostication are warranted.
Hong:Merck: Consultancy. Advani:Bristol Myers Squibb: Other: Consultancy/Advisory role and Institutional Research Support; Kyowa: Other: Consulting/Advisory Role; Infinity: Other: Institutional Research Support; Pharmacyclics: Other: Institutional Research Support; Seattle Genetics: Other: Consultancy/Advisory role, Institutional Research Support; Regeneron Pharmaceuticals, Inc.: Other: Institutional Research Support; Millenium: Other: Institutional Research Support; Bayer Healthcare Pharmaceuticals: Other: Consultancy/Advisory Role; Autolus: Other: Consultancy/Advisory Role; Takeda: Other: Consultancy/Advisory Role; Celgene: Other: Institutional Research Support; Roche/Genentech: Other: Consultancy/Advisory Role, Institutional Research Support; AstraZeneca: Other: Consultancy/Advisory Role; Cell Medica: Other: Consultancy/Advisory Role; Gilead/Kite: Other: Consultancy/Advisory Role; Agensys: Other: Institutional Research Support; Kura: Other: Institutional Research Support; Forty Seven, Inc: Other: Institutional Research Support; Merck: Other: Institutional Research Support; Janssen Pharmaceutical: Other: Institutional Research Support. Gascoyne:NanoString: Patents & Royalties: Named Inventor on a patent licensed to NanoString Technologies. Witzig:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kahl:Acerta: Consultancy; Celgene: Consultancy; Abbvie: Consultancy; Juno: Consultancy; CTI: Consultancy; Gilead: Consultancy; Seattle Genetics: Consultancy; Genentech: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy. Evens:Affimed: Consultancy; Novartis: Consultancy; Acerta: Consultancy; Bayer: Consultancy; Tesaro: Research Funding; Pharmacyclics International DMC: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal